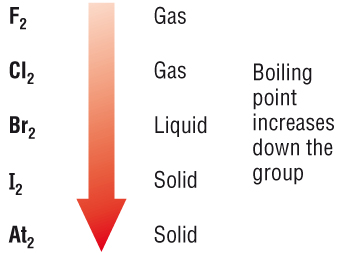
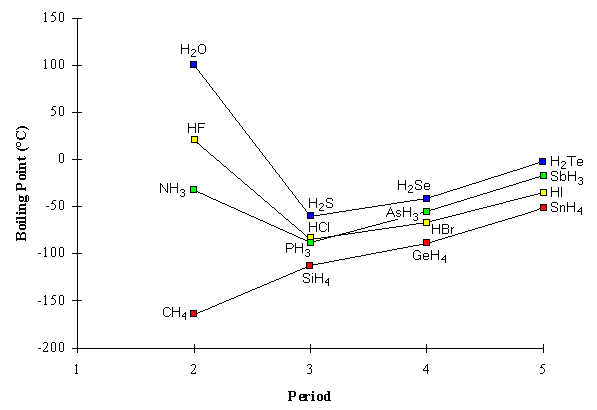
Which Compound Has a Higher Boiling Point? Intermolecular Force Boiling Point Relationship, Examples - YouTube

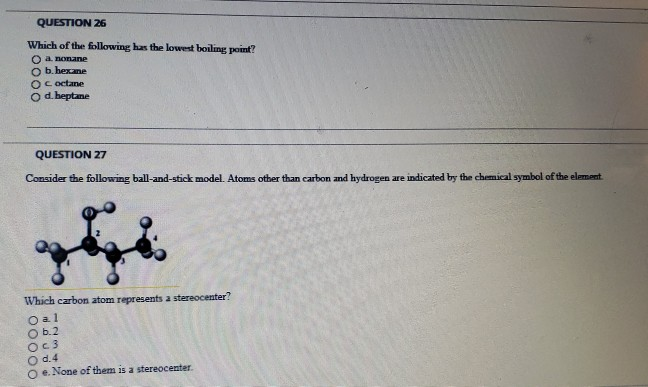
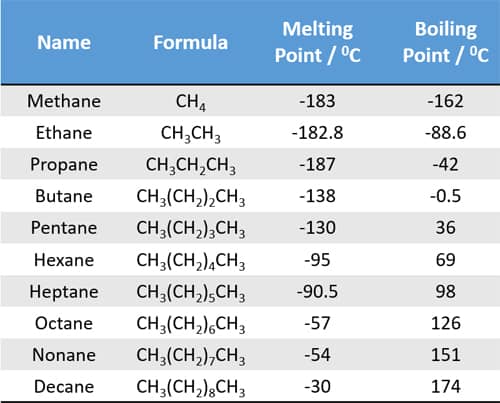
intermolecular forces - How can I determine the highest boiling point given a list of molecules? - Chemistry Stack Exchange

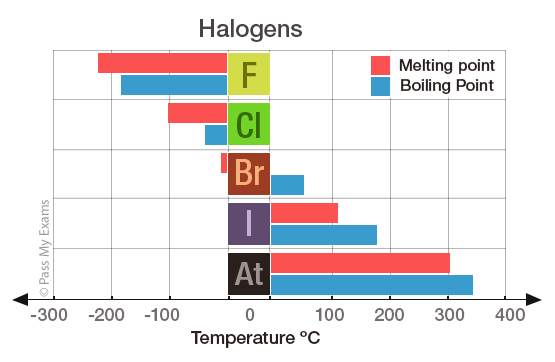
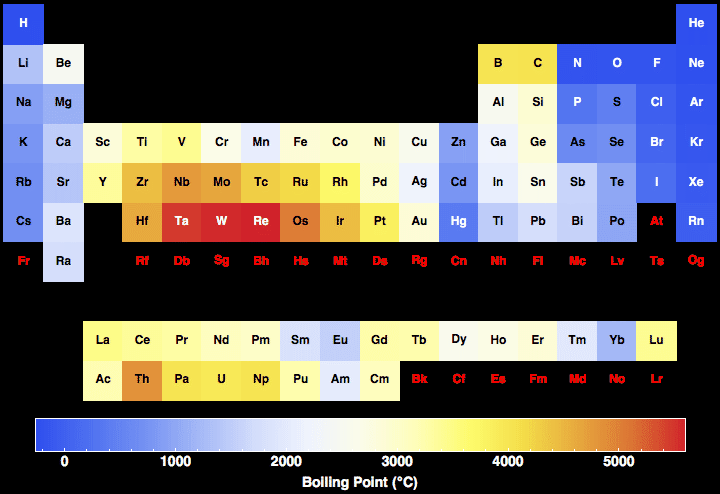
By: Alyssa Parsons. Helium has the lowest melting point and boiling point of the elements, so it only exists as a gas except under extreme conditions. - ppt download
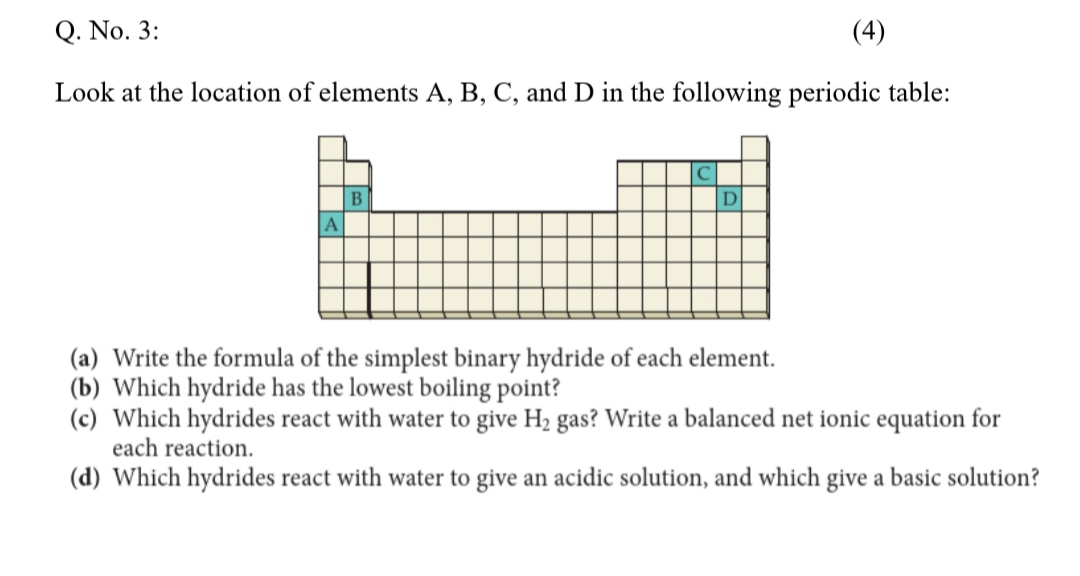
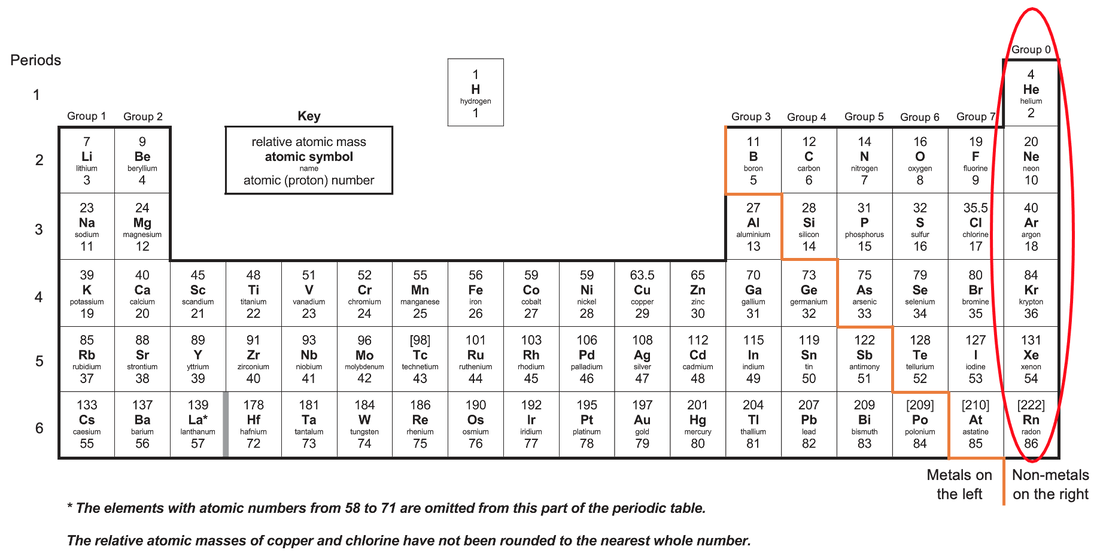
SOLVED: Q. No. 3: Look at the location of elements A, B, C, and D in the following periodic table: (a) Write the formula of the simplest binary hydride of each element. (

Why do the melting point and boiling point of transition metals high? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium