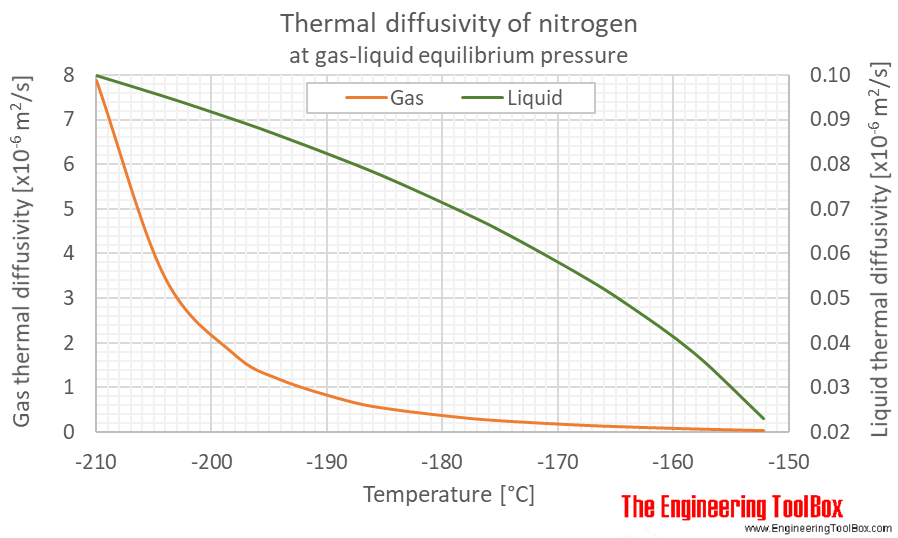
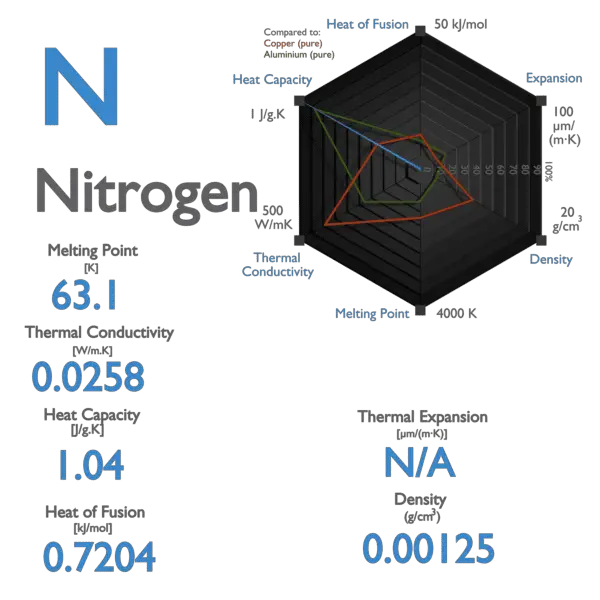
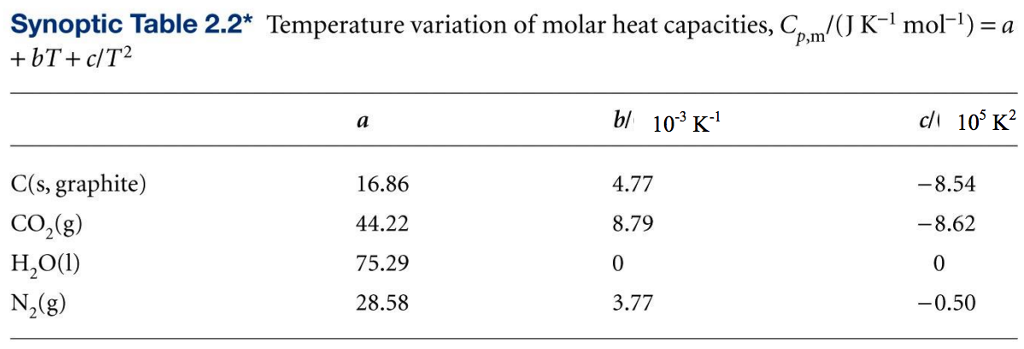
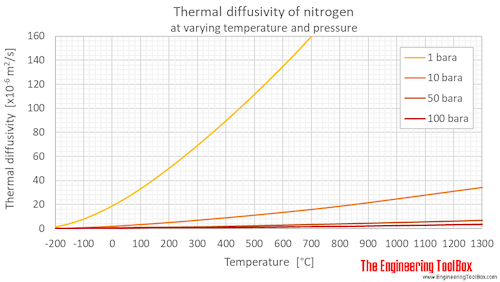
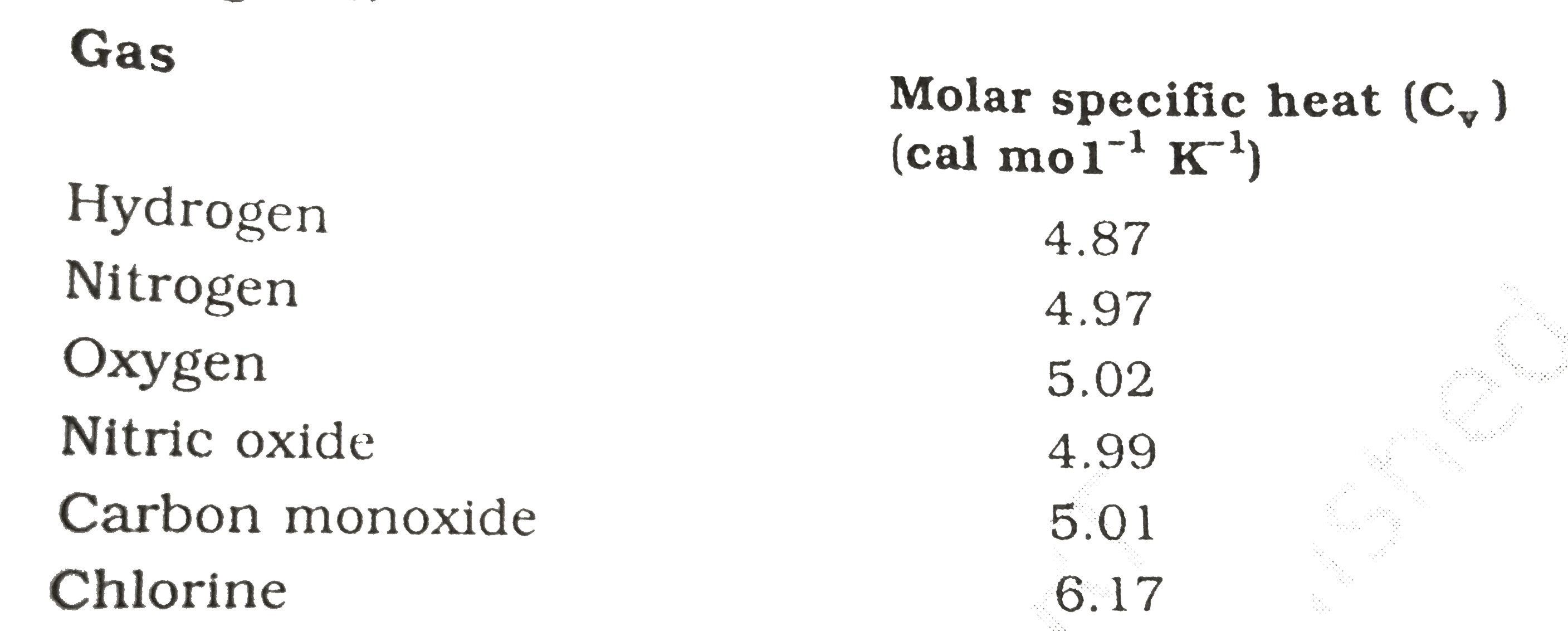The Molar heat capacities of nitrogen at constant pressure and constant volume are 29.11 kJ/k mole K and 20.81 kJ/kmole k, respectively. When 5 gram of nitrogen is heated from 290 to

The molar heat capacity at constant pressure of nitrogen gas at `STP` is nearly `3.5 R`.Now when the - YouTube

The temperature of 3 kg of nitrogen is raised from 10 ^o C to 100 ^o C. Compute the difference in work done if heating were at constant pressure and at constant

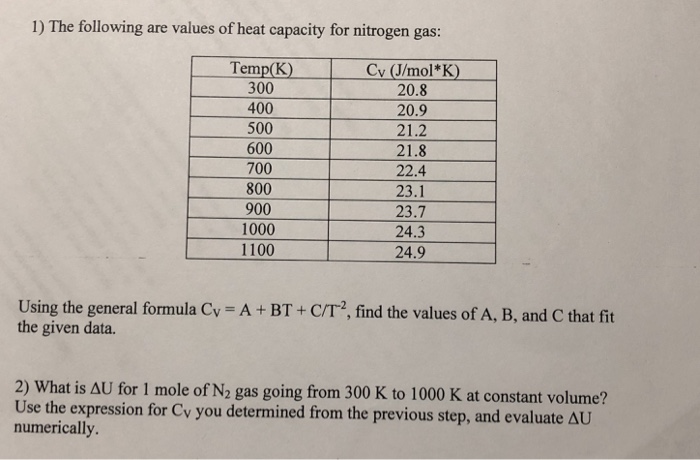
Density and specific heat of nitrogen as function of temperature. Data... | Download Scientific Diagram

This week in the physics course Lectures will cover Chapter 16 (Temperature and Heat) and start Chapter 17 (Thermal Behaviour of Matter) Tutorial class. - ppt download

Table 4 from Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 MPa | Semantic Scholar

Table 1 from Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 MPa | Semantic Scholar