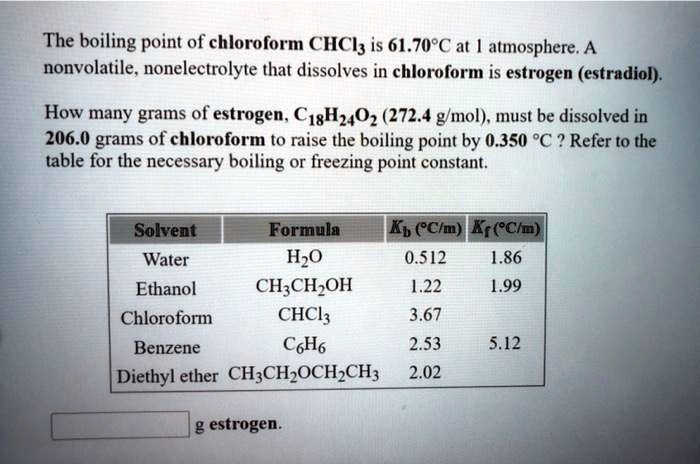
SOLVED: The boiling point = of chloroform CHCly is 61.70P€ at atmosphere. A nonvolatile, nonelectrolyte that dissolves in chloroform is estrogen (estradiol) How many grams of estrogen, C18Hz40z (272.4 g/mol) , must
Which one of the following form maximum boiling point azeotrope ? (1) CHCl3 + C2H5OH (2) Ethanol + water (3) Benzene + toluene (4) HCl + water Why option 1 is incorrect?
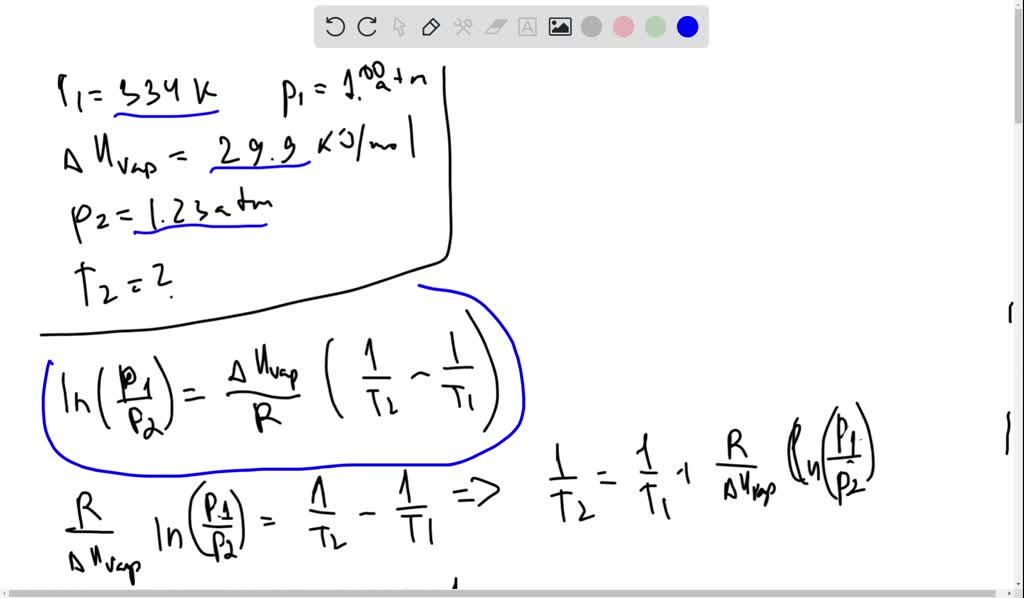
SOLVED: The normal boiling point of liquid chloroform is 334 K. Assuming that its molar heat of vaporization is constant at 29.9 kJ/mol, find the boiling point of CHCl3 when the external
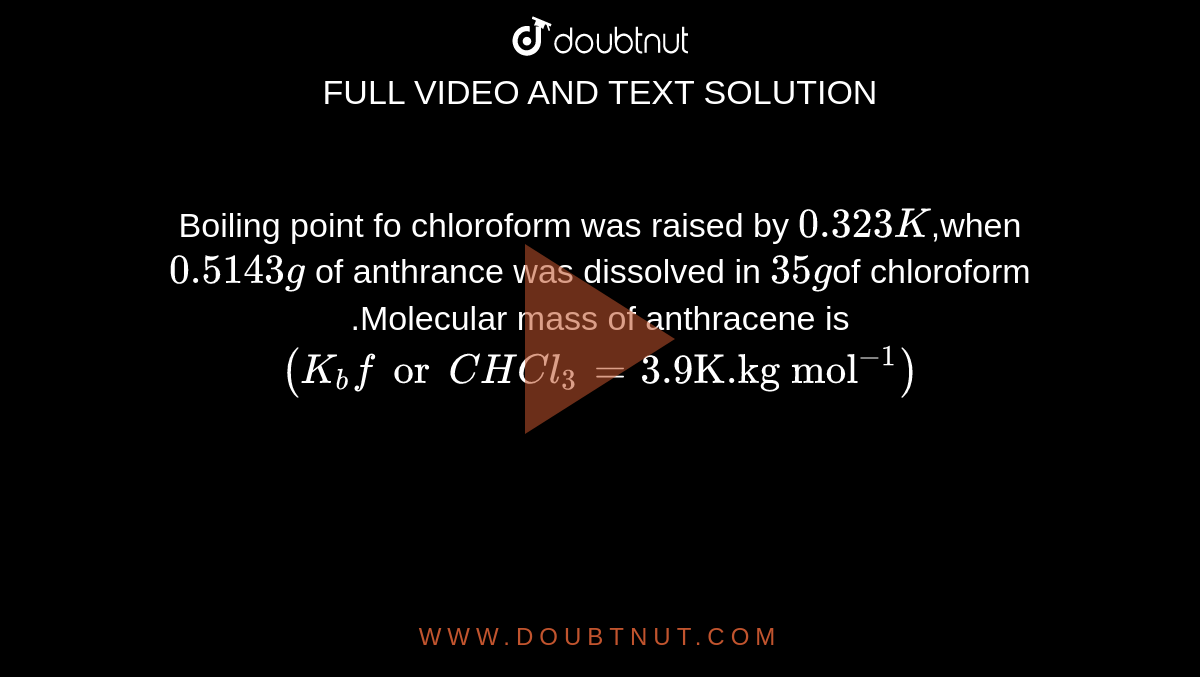
Boiling point fo chloroform was raised by 0.323K,when0.5143g of anthrance was dissolved in 35gof chloroform .Molecular mass of anthracene is (K(b)forCHCl(3)=3.9 "K.kg mol"^(-1))

Boiling point of chloroform was raised by 0.323 K , when 0.5143 g of anthracene was dissolved in 35 g of chloroform. Molecular mass of anthracene is ( Kb for CHCl3 = 3.9 K kg mol^- 1 )

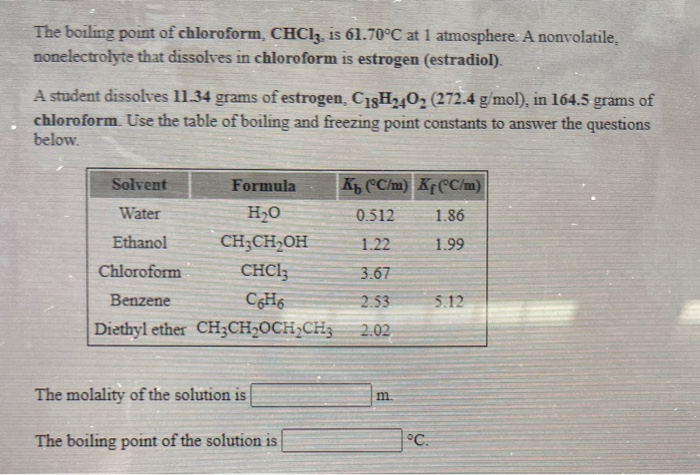
What would be the molar mass of a compound if 6.21 g of it dissolved in 24.0 g of chloroform form a solution that has a boiling point of 68.04^o C. The


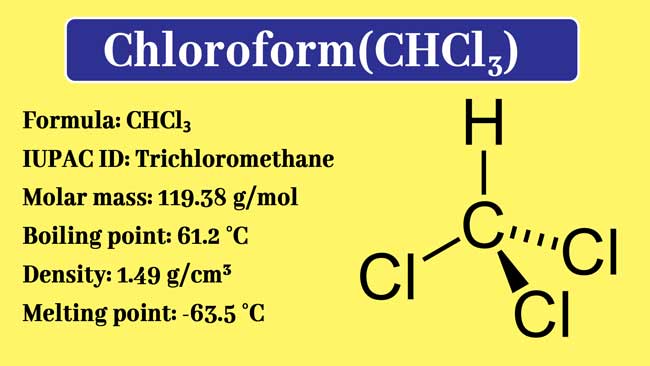




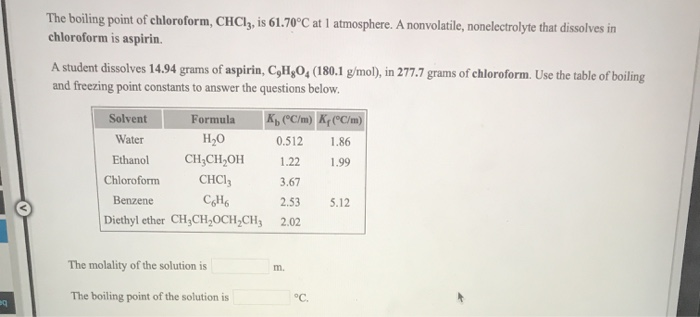
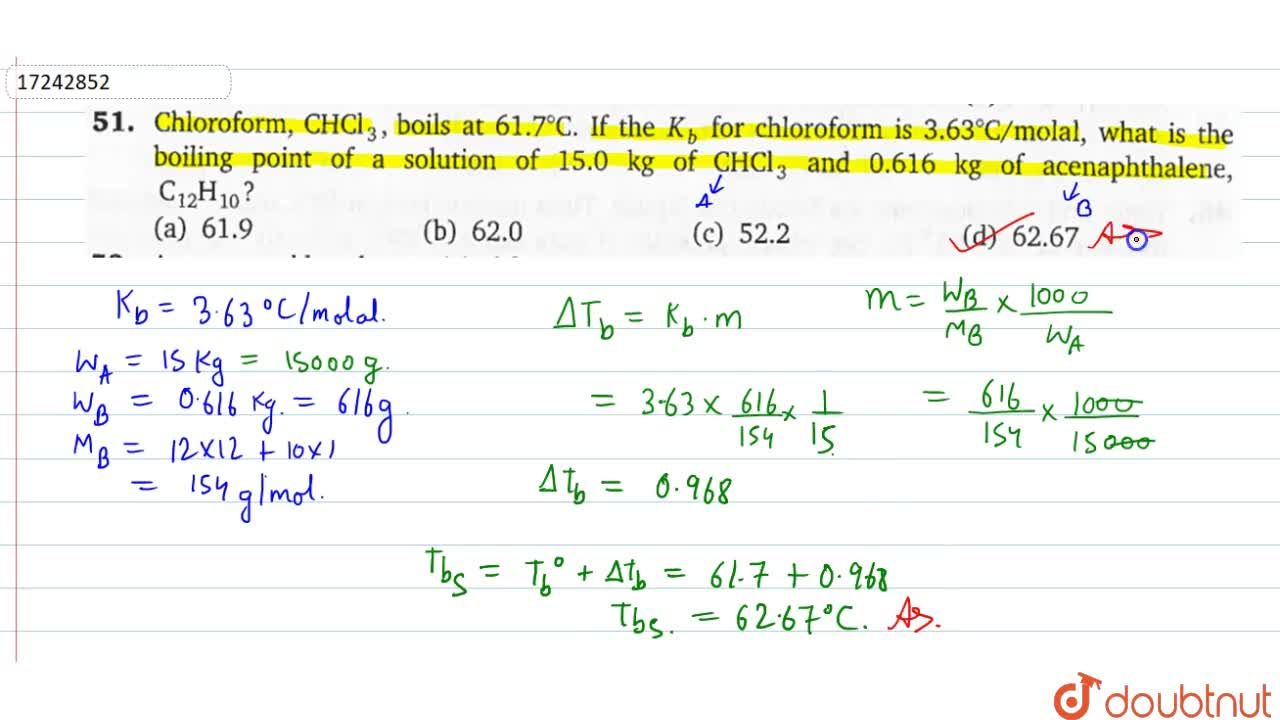
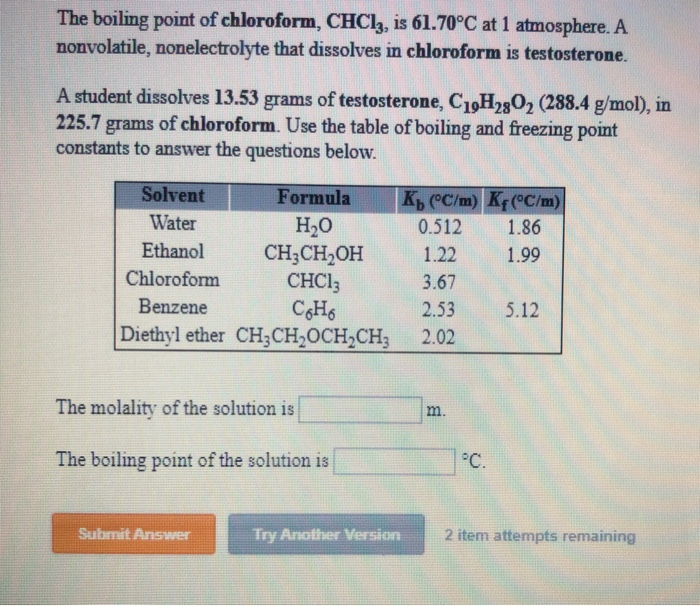



![Solved] Given the Graph Below,what Is the Boiling Point of Chloroform | Quiz+ Solved] Given the Graph Below,what Is the Boiling Point of Chloroform | Quiz+](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_6702_def6_93a6_659ab5e90581_TB6423_00.jpg)